Outforia Quicktake: Key Takeaways:
- Nitrogen is a fundamental component of life, despite being unusable by most organisms in its atmospheric form. It makes up around 78% (by volume) of the Earth’s atmosphere.
- Animals obtain nitrogen by consuming other living organisms. This typically involves herbivorous animals eating plants that have converted nitrogen into amino acids and proteins, and then carnivorous animals consuming the herbivores.
- The nitrogen cycle, involving processes like nitrogen fixation, nitrification, assimilation, decay, and denitrification, is a complex system that shifts atmospheric nitrogen into a form usable by living organisms.
- Nitrogen fixing plants, like the Legume or Pea family, facilitate this process by hosting Rhizobium bacteria, which helps convert nitrogen into a usable form.
- Nitrogen’s presence on Earth, as well as its roles in various natural and human-induced processes, are still subjects of extensive scientific research.

Nitrogen is a deceptively simple gas. It’s essential for life. It’s present almost everywhere we look. Yet it’s impossible for our bodies to use nitrogen until it has passed through a complex chain of reactions.
With that said, how do animals get nitrogen?
Animals get nitrogen by consuming other living things. They cannot get nitrogen directly, although it is present in 78% of our air.
Before plants and animals can use this atmospheric nitrogen, it needs to undergo conversion by bacteria or lightning.
How Do Animals Get Nitrogen?

Animals get nitrogen by consuming other living beings that have converted the nitrogen into a usable form. In most cases, this involves an herbivorous animal eating plants. Carnivorous animals then eat the herbivores.
Plants have nitrogen available in their tissues in the form of ammonia, which they have converted into amino acids and proteins. These can be used by animals that eat them.
In a marine environment, these plants may be very small, like one celled marine phytoplankton or algae.
Phytoplankton is eaten by shrimp, snails, and different species of jellyfish. These are then in turn eaten by larger marine animals such as herbivorous fish, which are eaten by carnivorous fish. Diatoms are a type of phytoplankton. You can see them below.
Fungi get nitrogen by consuming dead and decaying plants and animals.
What is Nitrogen?

Nitrogen is a colorless, odorless gas. It’s the most plentiful element in the Earth’s atmosphere. Around ⅘ of the Earth’s atmosphere is nitrogen. You can find nitrogen in all living matter. It is an element.
Nitrogen is in Group 15 in the periodic table. The periodic table lists all of Earth’s elements and sorts them according to their qualities.
Nitrogen is an inert gas. This means it doesn’t react with other gases or substances, as, for example, a gas like hydrogen would.
It’s safe to use in industrial processes, as it won’t explode. However, nitrogen is regarded as a volatile element because it can react with other elements to create compounds.
Why is Nitrogen Important?

Nitrogen is a core component of amino acids and proteins in animal bodies. It is needed to manufacture these. It’s needed to build DNA and RNA too, which are the building blocks from which all living things are made.
Plants also need nitrogen to complete their essential processes, such as photosynthesis. This is why nitrogen is added to fertilizers.
Nitrogen is essential for keeping plant leaves green. Without nitrogen, you will see that plants go yellow as they lose chlorophyll and start to die.
Without DNA and RNA, life would not exist. Without plants producing chlorophyll, there would be no oxygen for us to breathe.
I think we can safely agree that nitrogen is pretty important.
Where is Nitrogen Found?

You can find nitrogen in these places:
- The atmosphere, as a gas
- As part of amino acids, RNA, DNA and proteins
- In plant fertilizer
- In the atmosphere of some other planets or moons
- In the industrial processes detailed below
Nitrogen is used in so many industrial processes that it’s impossible to list them all here. Check out what humans use nitrogen gas and liquid for below.
You can find nitrogen as part of air and as part of complex molecules such as amino acids pretty much everywhere. To find pure nitrogen, you need to look for it in industry.
Nitrogen in nature is combined with other gases and molecules to form compounds as part of the nitrogen cycle.
Nitrogen in the Atmosphere
There is approximately 78% nitrogen and 21% oxygen in the atmosphere around us. There are trace amounts of other gases too, like neon, argon, CO2 (carbon dioxide), and hydrogen. So nitrogen makes up the vast majority of the air that we breathe.
It’s this ratio of nitrogen and oxygen that our bodies have evolved to breathe. We can’t breathe nitrogen on its own. That would lead to suffocation, as it would replace much needed oxygen. Breathing pure oxygen would damage our lung tissues. So, we need both for survival.
How Did Nitrogen Appear on Earth?
Nitrogen, along with hydrogen, methane, carbon dioxide and other volatile elements, has been present since the seeds of the rocky planets were formed. Some of the elements arrived as meteorites.
Most of our nitrogen came from the inner Solar System. Only a small part came from beyond Jupiter (the Outer Solar System). This is according to new research carried out by Damanveer Grewal and a team from Rice University, as part of a study by NASA.
Other studies by Prof Jabrane Labidi and Prof. Edward Young of UCLA have attempted to find out how much of our nitrogen comes from the Earth’s mantle through volcanic eruptions. Scientists still don’t fully understand how the Earth got its nitrogen.
Labdi, one of the paper’s authors, states that much of the nitrogen they found in volcanic eruptions actually comes from the atmosphere.
“Basically, air is contaminating the volcanic gases,” they tell us.
So it looks like nitrogen doesn’t come from within the Earth but rather from outside it.
Plants that Fix Nitrogen

Most plants need to wait until soil or marine bacteria break down nitrogen gas into soluble compounds. Some, however, are nitrogen fixing. This means they provide a home for Rhizobium bacteria.
Fabaecae family plants are known as the Legume or Pea family. These plants can fix nitrogen and use it.
Here are some Legume family plants that can fix nitrogen. They are great in your garden as they can make nitrogen available to other plants in your garden that can’t fix their own nitrogen.
- Peas
- Peanuts
- Soybeans
- Kudzu
- Lespedeza
- Alfalfa
- Clover
There are also shrubs and trees that can fix nitrogen. Here are some that are great nitrogen fixers and very pretty, or sometimes edible.
- Elaeagnus
- Broom
- Gorse
- Goumi
- Ceanothus
- Sea Buckthorn
These plants can fix nitrogen with the aid of special nodules, called nodes, on their roots. Different species of plants have different sizes and shapes of nodes.
Large nodes are more efficient at nitrogen fixation than lots of smaller nodes. Here you can see nitrogen fixing nodes on the roots of a Legume family plant.
The Discovery of Nitrogen

Here’s the timeline for the history of the discovery of nitrogen: It didn’t all happen at once.
1760: Henry Cavendish and Joseph Priestley discovered nitrogen in 1760 by removing all the oxygen from the air. They observed that once they did this, a lit candle would go out, and a mouse put in the container would soon die. Poor mouse.
1772: A young student called Daniel Rutherford worked out that nitrogen was an element in 1772. He published this as a thesis, earning the distinction of being the first person to recognize nitrogen.
1790: French chemist Jean-Antoine-Claude Chaptal named nitrogen in 1790. He named it after “nitre,” a mix of potassium nitrate (KNO3).
This is also known as “saltpetre.” This is because nitrogen was found to make up part of nitre.
The image above are some rocks containing saltpetre.
You may also like: 24 Types of Clouds (Facts, Photos and Chart)
The Nitrogen Cycle
The nitrogen cycle is the process by which atmospheric nitrogen is converted into a usable form for living organisms. There are several stages to this process. These are:
- nitrogen fixation
- nitrification
- denitrification
- decay
- putrefaction
The nitrogen cycle is constantly in action around us. We’ll now go through all these processes in more detail.
1. Nitrogen fixation

Here, atmospheric nitrogen is converted into ammonia (NH3). This happens mainly from precipitation (rainfall) into surface water and soil. Diazotroph bacteria are the ones responsible for this process. Azotobacter and Rhizobium bacteria are also at work here.
These bacteria contain a nitrogenase enzyme. This combines nitrogen gas with hydrogen and forms ammonia. This can also occur naturally through lightning hitting the earth or through man made processes such as manufacturing fertilizer.
The types of nitrogen fixation are:
- Atmospheric: By lightning and storms.
- Industrial: Manufacturing ammonia by combining nitrogen and hydrogen.
- Biological: Blue green algae and Azotobacter and Rhizobium bacteria can convert nitrogen gas to compounds that can be used within soil and water.
The photo above shows the blue green algae pollute a water source. This occurs when there are too many nitrates in the water. This can happen from fertilizer runoff or factory pollution.
2. Nitrification

Nitrification is when other types of bacteria turn the ammonia into nitrites. Nitrosomonas bacteria are responsible for this. After this, yet another type of bacteria, Nitrobacter, converts the nitrites into nitrates.
Nitrites are an intermediate product of the nitrogen fixation process. They need to be broken down further to be of use. They are used in the food industry to preserve food and add flavor. They are also used to cure meat.
Without nitrification, ammonia would remain in a form that is toxic to plants. So you see how important bacteria are to this vital process. Without them, life as we know it would not exist.
3. Assimilation

Plants take in the compounds produced by ammonia using their roots. Plants are known as primary producers. Now that the nitrogen has been converted into usable compounds, it has entered the food chain. Animals can now use it by eating the plants.
The giraffe is a link in a food web. It may be eaten by a predator such as a lion. Often, there are many more stages in a food web. A caterpillar may eat the plant, and be eaten by a bird, which is eaten by a fox.
4. Ammonification/Decay

When animals and plants die, their bodies are broken down. Fungi and bacteria are in charge of this step of the process. They break the living matter down into ammonium, which can be used to produce ammonia.
Ammonia has that characteristic smell like a sharp tang of decay. On its own, it forms a caustic acid when mixed with water. In animal bodies, it is used to produce proteins and other complex molecules.
We know this process better as decay. Decay is not a bad thing. It’s simply part of the nitrogen cycle that sustains all life.
5. Denitrification
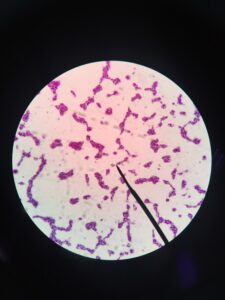
This final stage of the process is carried out without oxygen – in anaerobic conditions. Again, bacteria are at work here. This time, it’s Clostridium and Pseudomonas. They convert nitrates back into nitrogen gas. They do get something out of this – oxygen, which they use. Nitrogen gas is a by-product of this process.
In the marine ecosystem, the nitrogen cycle operates in much the same way. It’s just that marine bacteria are responsible for the processes instead of land based bacteria.
On the image, you can see Clostridium botulinum, which is toxic. It’s this group of Clostridium bacteria which are vital for life’s processes too.
Check out the video below to learn more about the Nitrogen Cycle:
FAQs

Are there any animals that can convert nitrogen gas from the atmosphere?
Animals cannot convert nitrogen into usable form directly. Instead, they must consume plants or other animals which have nitrogen fixed in the form of proteins and amino acids.
Is the nitrogen cycle different in water?
The nitrogen cycle operates in exactly the same way as it does on land. However, there are marine bacteria that carry out the processes instead of land based bacteria.
Can breathing pure nitrogen kill you?
Breathing pure nitrogen with no oxygen in it would lead to suffocation. This would have the same effect as breathing pure CO2 (carbon dioxide).
Is there nitrogen on other planets?
There is nitrogen on other planets. In contrast to Earth’s 78% nitrogen, Mars has only 2.6% nitrogen in its atmosphere.
Pluto has an atmosphere that’s 90% nitrogen. Titan, a moon, has a 95% nitrogen atmosphere. All the other planetary bodies in our Solar System have no or low amounts of nitrogen.
Is liquid nitrogen dangerous?
Liquid nitrogen is dangerous. It needs to be handled with care, as it can cause burns on exposed skin or if drunk.
It also expands rapidly from a small volume of liquid into a large volume of nitrogen gas, displacing the oxygen in a confined space. This can cause suffocation.